Redefining the Potential of Immunotherapy
Dendritic
Cell Therapy
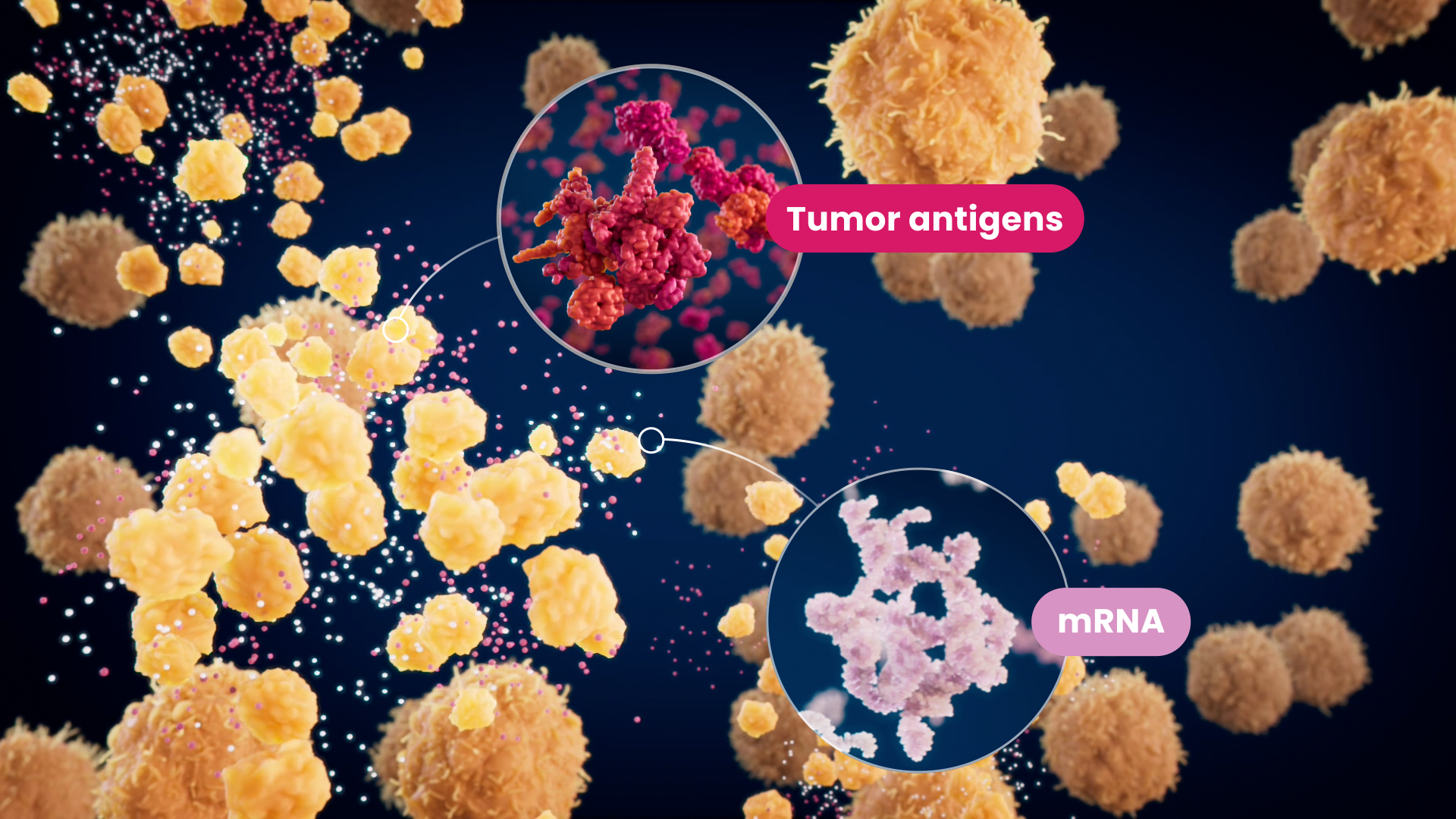
Developed at the Texas Medical Center, DOC1021 represents a novel approach to fighting cancer that harnesses the body’s natural anti-viral immune response.
At the core of DOC1021 is Diakonos’ proprietary “double-loading” technique. This patented process tricks the body into perceiving cancer cells as virally infected cells, mirroring the natural detection and elimination process for viral infections.
Learn More
At the core of DOC1021 is Diakonos’ proprietary “double-loading” technique. This patented process tricks the body into perceiving cancer cells as virally infected cells, mirroring the natural detection and elimination process for viral infections.
Powered by a
Groundbreaking
Discovery on the
Immune System
DOC1021 is based on a discovery on how dendritic cells are able to detect and respond to viral threats. Based on Diakonos’ research, it is now known that dendritic cells compare antigens presented on MHC Class 1 and 2.
When identical antigens are detected on both MHC Class 1 and 2, dendritic cells mobilize formidable CD8 CD161 T cells to destroy what the immune system believes are infected cells throughout the body.
Learn More About Our Research
When identical antigens are detected on both MHC Class 1 and 2, dendritic cells mobilize formidable CD8 CD161 T cells to destroy what the immune system believes are infected cells throughout the body.
Diakonos Oncology
News & Media
Clinical Trials
Underway
Discover our treatment centers and find out which one is best for you.





